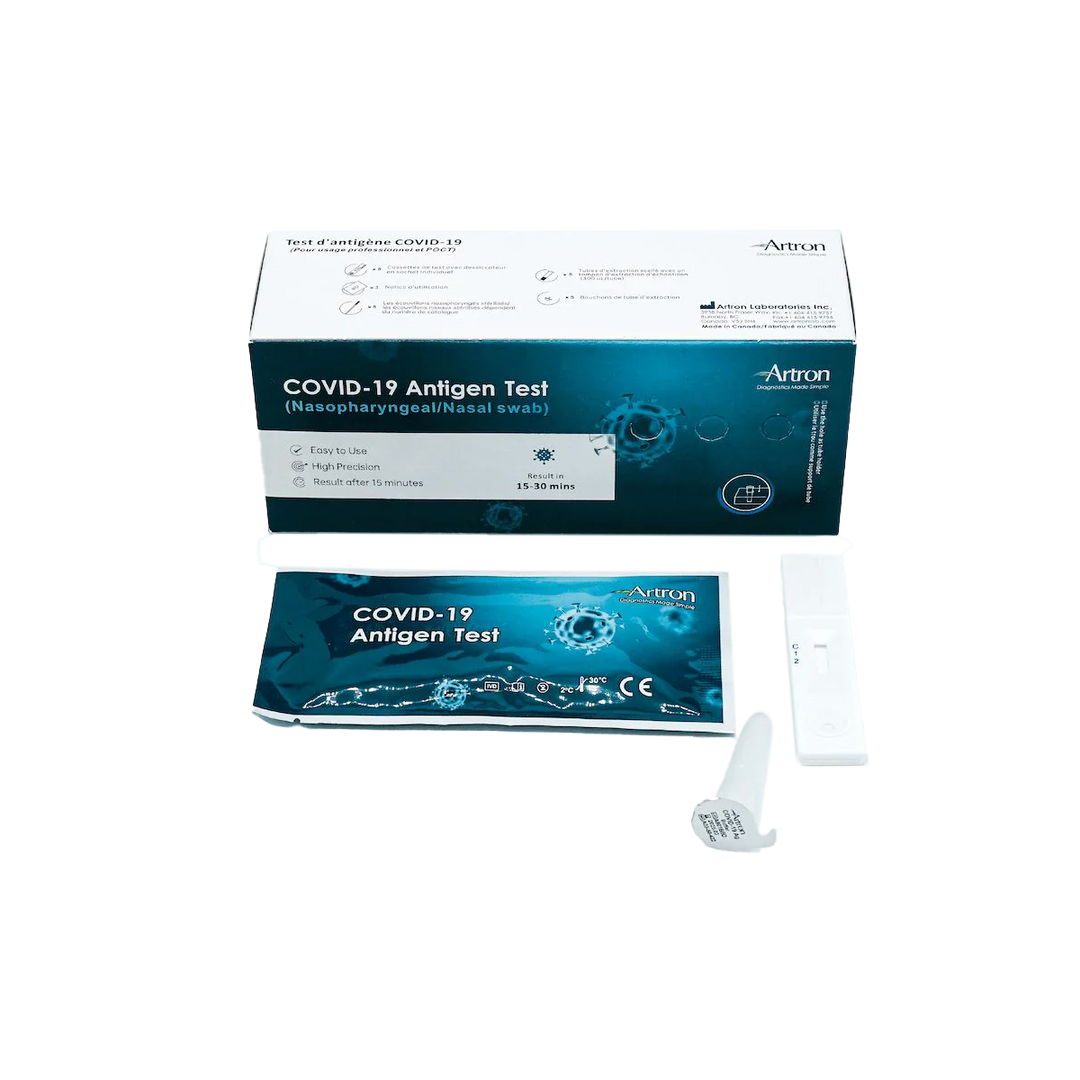
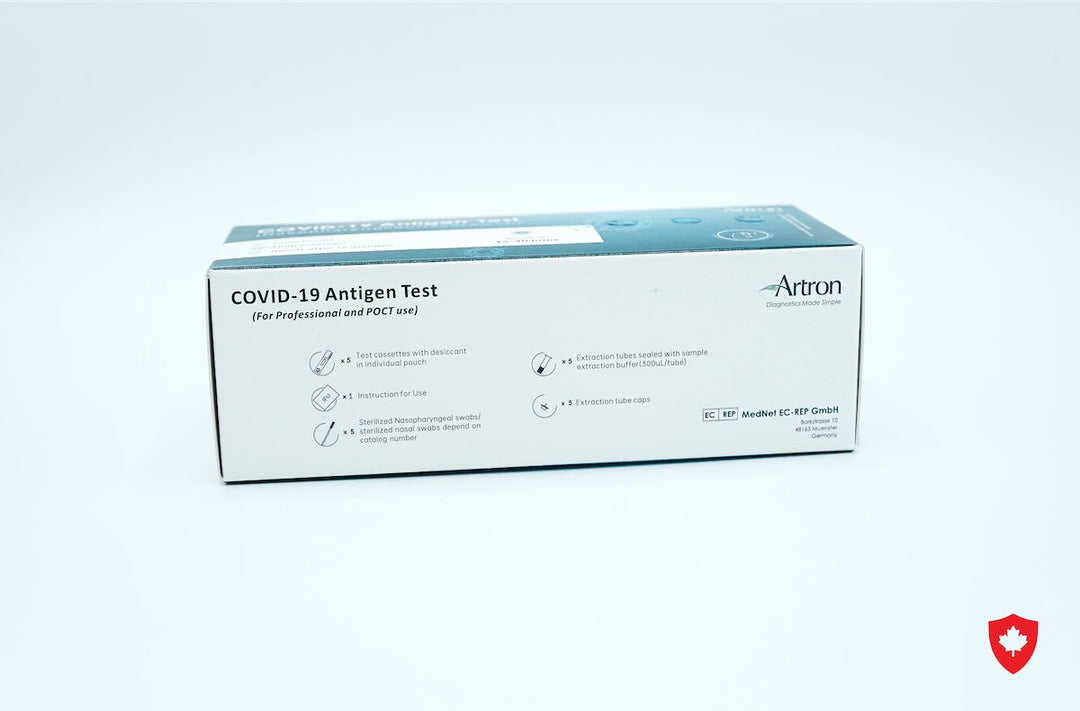
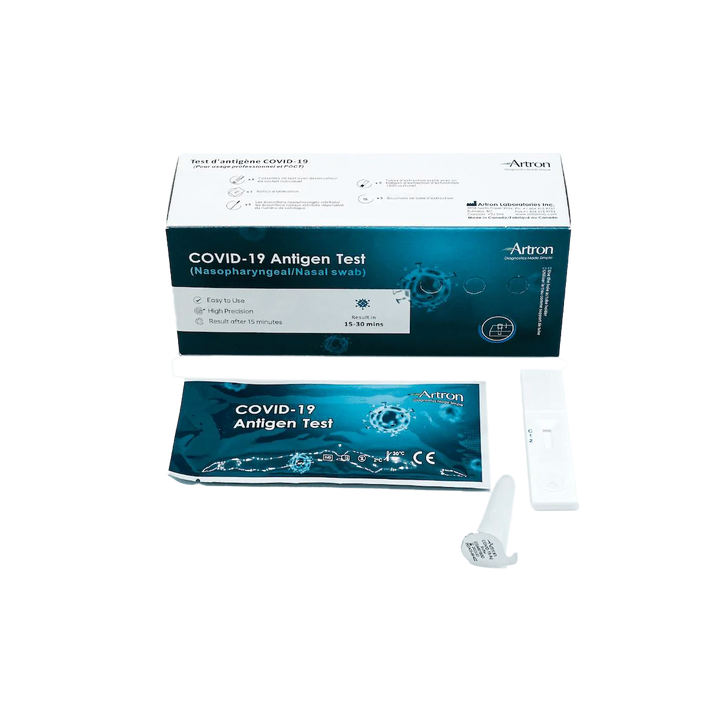
The Artron COVID-19 Antigen Test is authorized for healthcare professional use by Health Canada (Interim Authorization Number: 321669). Before Rapid Testing kits can be purchased, the following training materials and documents must be reviewed:
Artron Laborations Rapid Antigen Test Instruction VideoArtron Rapid Antigen Test Kit Brochure and ManualArtron Rapid Antigen Test Kit Instructions for Use
Rapid antigen testing devices should only be used for screening purposes. Nucleic acid-based testing, also called molecular testing or PCR, is the gold standard to diagnose active COVID-19 infection in patients with symptoms. For more information about self-testing and antigen testing devices, please visit the Health Canada website here.
General Information about the Artron COVID-19 Antigen Test
The Artron COVID-19 Antigen Test is an easy-to-use rapid test for the qualitative detection of COVID-19 antigen (viral nucleoprotein) from swabs obtained from patients with signs and symptoms of respiratory infection.
Cold Weather Warning
As the weather gets colder in Canada, we understand some customers may be concerned about the sub-zero temperatures during the shipment of Rapid Test Kits. Transport temperatures dipping below 0°C for short durations of time will not have an adverse effect on the test, or the buffer liquid.
Recommendation: It’s important to bring the tests to room temperature before adding the sample into the test, as low ambient temperatures when conducting the test may cause false results. We recommend letting the test kit contents sit out for 4-6 hours before use.
** All Rapid Tests and PPE are FINAL SALE - we cannot offer cancellations or refunds after your order has been fulfilled or shipped. **
All orders from The Canadian Shield are fulfilled in 1-3 business days from our facility in Waterloo, Ontario (unless otherwise specified for product pre-orders).
Standard Shipping - delivery within 3 to 9 business days after fulfillment.
EXPRESS Shipping - delivery within 1 to 2 business days after fulfillment.
All orders will be accompanied with a tracking number at the time of fulfillment. Shipping costs are calculated at checkout.
Health Canada Authorized
Authorized by Health Canada for nasal and nasopharyngeal swabbing.
Results in 15-30 Minutes
Easy-to-use test with instructional videos and easy-to-follow diagrams.
Most Affordable Prices
The Canadian Shield offers the most affordable rapid test prices in Canada.
Instructions & Demonstration
Please watch the video below for guidance on how to collect nasal specimens and administer the Atrton COVID-19 Rapid Antigen Test.
Instructions for Use
Written product manual and instructions for use.

Health Canada
ANTIGEN TESTING DEVICES INFORMATION & RESOURCES
Information, regulations, and guidance around the use of rapid antigen testing devices in Canada is frequently changing. While The Canadian Shield website is updated often, we recommend referring to the Health Canada website for the most up to date information on rapid antigen testing in Canada.
Rapid Test FAQs
Yes! The Canadian Shield holds a Medical Device Establishment License from Health Canada.
The Artron COVID-19 Rapid Antigen Test is a Health Canada authorized COVID-19 testing device for Point of Care testing. The Artron COVID-19 Antigen Rapid Test Device is intended for use by trained laboratory personnel or health care professionals.
For a full list of authorized testing devices, please visit the Health Canada website.
Rapid Tests are used as a serial screening tool whereby individuals test themselves 2 or 3 times a week to detect if they are an asymptomatic carrier of covid-19. These tests are most effective when you are infectious and are one more layer of peace of mind and safety.
Rapid antigen testing devices should only be used for screening purposes. Nucleic acid-based testing, also called molecular testing or PCR, is the gold standard to diagnose active COVID-19 infection in patients with symptoms. For more information about self-testing and antigen testing devices, please visit the Health Canada website here.
Yes. Like all medical devices, the Rapid Response COVID-19 Rapid Antigen Testing device has an expiration date listed on the outside of the kit box as well as the Cassette testing device package.
The Rapid Antigen Test kits sold by The Canadian Shield are not intended for travel.
The regulations around COVID testing for travel is highly dependent on the country you are traveling to. While rapid antigen tests may be accepted in some countries, these tests need to be performed and signed-off by a trained individual, doctor, or pharmacist. We highly recommend you inquire with your country of destination or airline to find out what type of test you need to travel.
The BTNX nasal swabs are sterilized using ethylene oxide. This process is completely safe for the user and has been used for decades on medical equipment. The sterilization process follows ISO 11135 (Sterilization of health-care products) and ISO 10993 (Biological evaluation of medical devices). Additionally, all nasal swabs have passed testing for both toxicology and biocompatibility.
Rapid tests should be stored in a cool, dry place. Please refer to the side of the rapid test kit packaging for the exact temperature requirements.
As the weather gets colder in Canada, we understand some customers may be concerned about the sub-zero temperatures during the shipment of Rapid Test Kits.
Transport temperatures dipping below 0°C for short durations of time will not have an adverse effect on the test, or the buffer liquid.
Recommendation:It’s important to bring the tests to room temperature before adding the sample into the test, as low ambient temperatures when conducting the test may cause false results. We recommend letting the test kit contents sit out for 4-6 hours before use.
Yes, there is tax charged on rapid tests! While there is a zero-rated tax exemption on PPE products (such as face shields and face masks), there is currently no zero-rated tax exemption for rapid testing devices in Canada.
If the appropriate test procedure and result interpretation has been followed and the result of your rapid antigen test is positive – contact your healthcare providers. You will need to have a second swab taken within 48 hours with a regular laboratory-based PCR test or a rapid molecular test. This may occur at a designated testing site. The result from this test would confirm if you truly tested positive for COVID-19. Continue to self-isolate while waiting for the laboratory PCR test result.
You may collect your sample using either method, however collecting an anterior nasal swab only requires you to insert the swab 0.5 inches (1-2cm) into the nostril making it a simpler method of collection and leading to less discomfort that the nasopharyngeal collection. Both the nasopharyngeal and anterior nasal swab collection methods are capable of being used for rapid antigen testing to detect the SARS-CoV-2 nucleoprotein.
Compared to the regular laboratory-based PCR test, COVID-19 rapid antigen tests must be considered preliminary. The results from the antigen test must be confirmed with a regular laboratory-based PCR test. Interpretation of results in different populations varies based on specimen type collected and pre-test probability of COVID-19 in the patient being tested.